Foods such as spinach and chard are advertised as healthy, but shockingly, research shows that they may predispose us to infection and chronic inflammatory disease. So, which is it—is spinach good or bad?
Patients already struggling with chronic illness, digestive problems, immune issues, or kidney stress may be especially vulnerable to the oxalate in foods like spinach— yet most providers assume that high oxalate foods are safe.
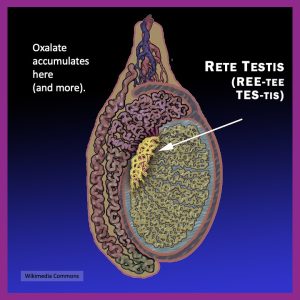
Oxalate is the chief constituent in kidney stones. Oxalic acid and the calcium crystals it forms damage the kidneys, which is how stones grow and get stuck there on their way out of the body. Doctors think we tolerate oxalate unless we’re prone to kidney stones. But that assumption is dangerous. Oxalate can impact health far beyond the urinary tract—and in anyone—because it tends to damage and impair most any cell it encounters, including immune cells.
Given the popularity of not just “superfoods” like spinach smoothies, but oxalate forming high-dose vitamin C and collagen supplements, the risks of overloading the body with oxalate are no longer rare or theoretical. Oxalate overload is here, and hardly anyone is noticing its wreckage.
How Does Oxalate Weaken the Immune System?
Excessive oxalate has multiple effects that make it toxic. It directly and indirectly impairs our natural immune defenses by disrupting intracellular control, damaging circulating immune cells, inflaming mucosal tissues, and driving chronic oxidative stress. At the same time, it allows pathogens to thrive by altering the gut microbiome.
Too Much Oxalate
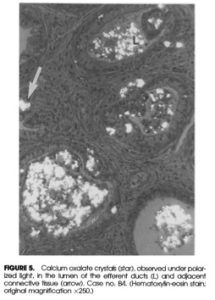
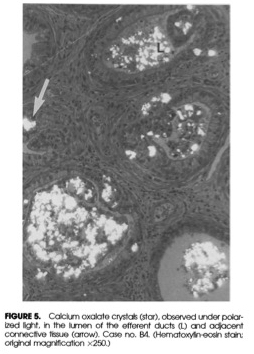
Oxalic acid occurs naturally in most plant foods and must be eliminated from the body — primarily via the kidneys. The oxalic acid in nuts, kiwi, potatoes, beets, chia, and other foods readily moves into the bloodstream and tissues (Chen et al. 2003). It strongly binds calcium ions and forms calcium oxalate crystals throughout the body. Tissues that are injured, regenerating, infected, or where inflammation is already occurring are places where oxalate gets deposited.
Around 85% of oxalate in the body comes from diet and supplements (Holmes et al., 2001. Knight et al., 2016).
Food is not the only source of oxalate. One to four mg/day of oxalate is made in the body, principally from the amino acid hydroxyproline, a component of collagen. Unavoidably, tissue turnover and collagen ingestion generate endogenous oxalate, contributing 5–20% of total urinary oxalate (Knight et al., 2006).
Ingestion of more than 7 g of gelatin increases oxalate production. Oxalates are also produced when vitamin C (ascorbic acid) converts into oxalate within cells. Hatch et al. (1980) showed that megadoses of vitamin C (8 g/day) tripled blood oxalate and pushed urinary oxalate into the toxic hyperoxaluric range — especially after stopping supplementation. This means vitamin C may cause:
- Acute oxalate elevations after ingestion
- Tissue accumulation from chronic use
- Delayed release from tissues even after exposure is discontinued
- Lingering oxalate deposits causing chronic exposure
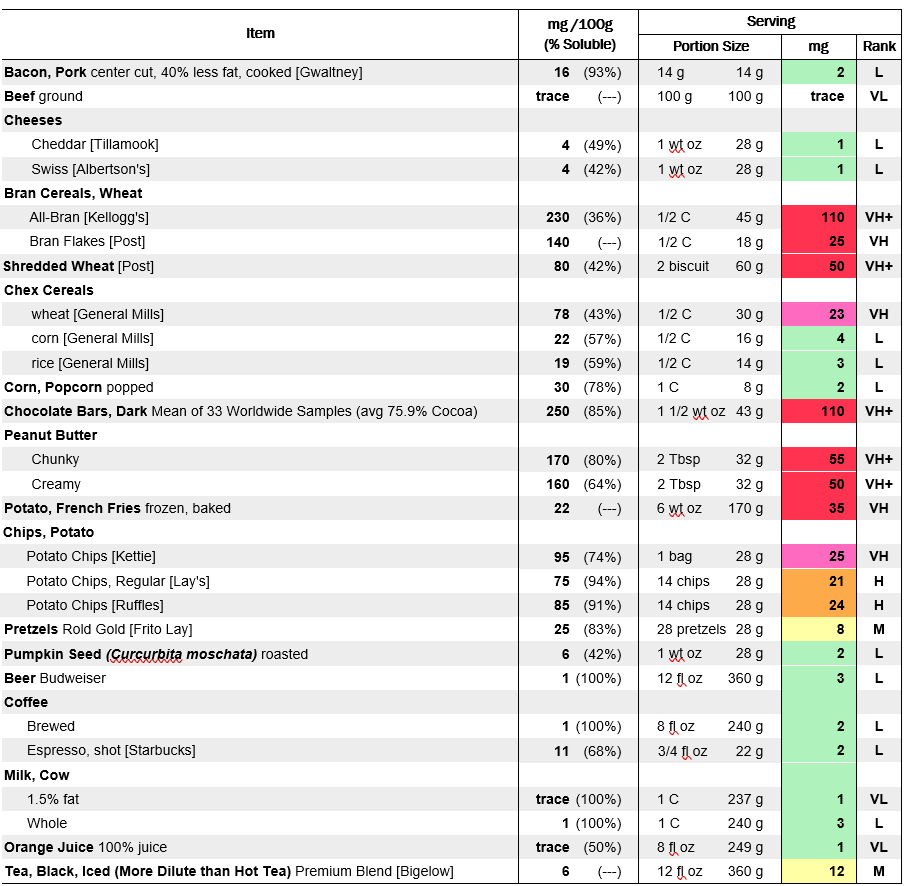
The kidneys can safely clear about 20–30 mg of oxalate daily. Because dietary oxalate moves into the bloodstream at about 10–15%, most healthy people can tolerate about 150–200 mg oxalate/day. Intakes of high-oxalate foods, such as a spinach smoothie (typically containing 700–1,200 mg of oxalate), can push urine oxalate to dangerous levels for several hours (Balcke et al., 1989).
Daily urinary oxalate loads come from dietary oxalate (~50%), vitamin C (~35%), and collagen breakdown (~15%) (Mashour, et al. 2000. Knight, et al., 2016).
With today’s emphasis on plant-based eating and high-dose vitamin C supplementation, and the popularity of collagen powders, oxalate exposure has quietly increased in many patient populations, which can easily elevate oxalic acid to toxic levels.
Damage From Nature’s Poisoned Spears
In addition to oxalic acid, plant foods contain hard calcium oxalate crystals in various shapes. Raphide crystals, shaped like thin, sharp toothpicks, are found in kiwi, pineapple, taro leaves and tubers, and wild yam (Savage & Dubois, 2006; Holloway et al., 2002). When raphides physically injure tissues, they cause a stinging burn in the mouth and sometimes gastrointestinal distress (Bhandari & Kawabata, 2003).
Perera et al. (1990) and Konno et al. (2014) showed how these mini “oxalate arrows” pierce cell membranes in a “needle effect,” with damage amplified by plant proteases such as bromelain in pineapple. Bradbury & Nixon (1998) aptly called them “Nature’s poisoned spear.”
In the gut, this kind of injury inflames and weakens the mucosal barrier, increasing susceptibility to infection and slowing recovery. Hatch (1998) also documented mucosal injury from dietary oxalate. Oxalate-induced gut problems can look like stubborn IBS, chronic diarrhea, or increased food sensitivities that fail to improve with standard dietary approaches.
[Note: Calcium oxalate crystal shapes in plant foods have not been systematically characterized.]
Oxalate Helps Pathogens Thrive
Injury is only part of the story.
When oxalate is elevated, the intestine excretes oxalate to remove excess from the blood (Robijn, 2011). In the colon, excreted oxalate may be degraded by various bacteria. Suryavanshi et al. (2016) found that high oxalate excretion is toxic to many common oxalate-degrading microbes, including Oxalobacter formigenes, and promotes the rise of acid-tolerant pathogens. This imbalance, combined with weakened gut cells and damage to the intestinal barrier, significantly inflames the intestines and undermines systemic immunity.
Oxalate Hampers Antibody Production
Calcium ions play a precise, timing-dependent role in immune cell fate and function. Immune cell activation and antibody production depend on calcium and the cells’ ability to maintain strict control over it (Diamantstein & Odenwald, 1974).
Oxalic acid causes intracellular demineralization (Beck, 1973). Because oxalate binds calcium so strongly, when it locally depletes essential calcium ions, it disrupts immune cell communication and impairs long-term immune memory formation, increasing the likelihood of repeated infections. In T cells disruptions in calcium ion control also contribute to diverse autoimmune, inflammatory, and immunodeficiency conditions (Diercks, 2024).
Oxalate Creates Inflammation
Oxalic acid is a toxic irritant that sets up crystalline foreign bodies in tissues. The resulting toxicity is likened to silica and asbestos particles. At each phase (ionic, nanocrystals, microcrystals), oxalate induces inflammation. Tissue stress provokes a para-inflammatory response from tissue-resident macrophages (Medzhitov, 2008). Although the body’s goal is repair, para-inflammation can lead to fibrosis, cancer, and other modern inflammatory diseases.
Calcium oxalate crystals can grow from nano-sized particles to large, sharp shards. These awkward shapes can cause “frustrated phagocytosis,” where immune cells cannot engulf them, leading to giant-cell and granuloma formation. In breast tissue, Scimeca et al. (2019) showed that oxalate crystals can drive cancer progression via macrophage-induced epithelial-to-mesenchymal transition.
Immune Cell Killer
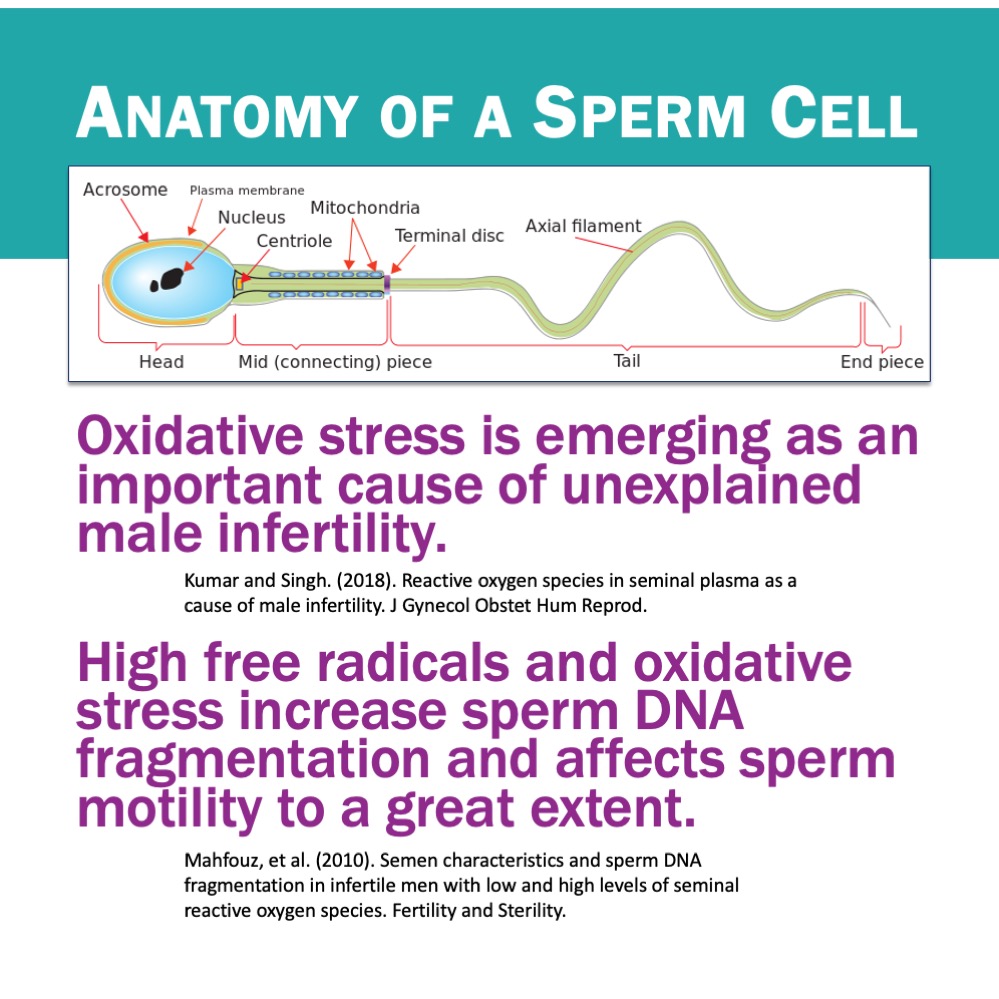
A 2021 study by Kumar et al. found that consumption of a high-oxalate spinach smoothie impaired mitochondrial function in circulating monocytes and macrophages. These cells produced less ATP, generated more reactive oxygen species, and shifted toward a pro-inflammatory but less effective state (higher IL-1β and IL-6, lower IL-10). Oxalate exposure also increased bacterial survival inside macrophages.
Consuming just one spinach smoothie weakened the ability to clear bacteria — directly linking oxalate exposure to infection and poor resolution. This could explain why some people struggle with recurrent UTIs, sinus infections, or poor wound healing despite otherwise “normal” immune labs.
In older lab studies, oxalate has been shown to interfere with several cellular enzymes that produce ATP energy (James, 1972). There are probably multiple ways in which oxalate impedes immune cells and destroys their function. Oxalate crystals are known to scramble cell membranes (Wiessner et al, 2001), with nanocrystals being the most toxic crystal size (Sun et al., 2015).
Oxalate Triggers Chronic Inflammatory Pathways
Elferink and colleagues (1980, 1987) demonstrated that calcium oxalate crystals activate neutrophils, cause enzyme release, and induce cytolysis in both immune and red blood cells.
Later, Ermer et al. (2016) confirmed that oxalate crystals activate the NLRP3 inflammasome, triggering the release of IL-1β — a key driver of chronic inflammation.
Oxalate could be an under-recognized factor in conditions like fibromyalgia, unexplained joint pain, or persistent fatigue syndromes.
Oxalate and Oxidative Stress: A Vicious Cycle
Oxalate-induced tissue injury promotes oxidative stress. Perera et al. (2018) reported that oxidative damage from oxalate exposure can perpetuate inflammation and impair mitochondrial recovery, creating a cycle of tissue injury and impaired repair mechanisms.
Oxalate Can Accumulate in Multiple Organs
Oxalate can deposit in tissues beyond the kidneys — including bone, spleen, liver, and heart (Blumenfrucht et al., 1986; Zimmerman et al., 2005; Marengo et al., 2013). Deposits in the bone marrow and spleen may directly interfere with the development and regulation of immune cells.
In veterinary medicine, Schiefer and Moffatt (1974) reported 175 cases of bovine miscarriage where oxalate from the mother’s diet built up in fetal kidneys, leaving the developing calf vulnerable to deadly infections. They speculated that pregnancies where oxalate poisoning did not cause fetal death still predisposed calves to infectious disease after birth.
This illustrates how oxalate’s disruption of cell function and tissue integrity can predispose us — even before birth — to immune failure.
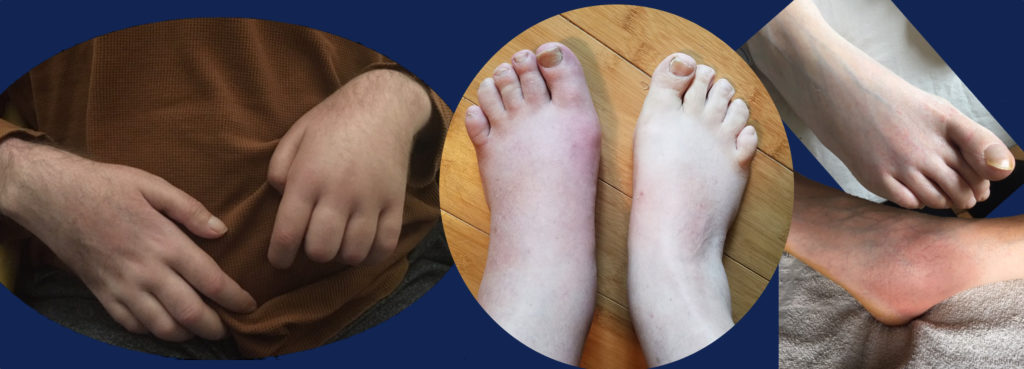
Human patients struggling with oxalosis may have overlapping symptoms such as fatigue, bone pain, anemia, low white cell counts, and immune dysregulation. These symptom patterns are often dismissed as “non-specific.” Pediatric patients may struggle with infections and any number of behavioral issues.
Practical Steps to Reduce Oxalate Burden
- Identify and moderate high-oxalate foods (e.g., spinach, almonds, sweet potatoes, turmeric, tea, beets, peanut butter).
- Avoid unnecessary high-dose vitamin C supplementation.
- Limit collagen and gelatin supplements to 2 tsp daily.
- Pair moderate-oxalate foods with calcium sources to reduce absorption.
- Consume adequate calcium and stay well hydrated to promote clearance.
Practical Takeaways for Providers
- Ask about high-oxalate foods (spinach, chard, almonds, beets, peanuts, dark chocolate, rhubarb) in dietary histories.
- Screen supplement use, especially high-dose vitamin C and collagen powders.
- Encourage pairing oxalate foods with calcium sources to reduce absorption.
- Support gut health — avoid unnecessary antibiotics that deplete oxalate-degrading bacteria.
- Be alert for oxalate overload in patients with chronic inflammation, unexplained immune dysfunction, or recurrent infections.
Conclusion
Oxalate is not just a kidney stone issue — it is a stealth driver of immune dysfunction, chronic inflammation, and vulnerability to infection. With spinach smoothies, almond milk, nut snacks, gluten-free diets, and high-dose vitamin C supplements now mainstream, oxalate overload is no longer a rare occurrence.
Yet, oxalate overload is easy to miss and rarely considered in routine clinical care. That’s because clinicians are not adequately informed. As a result, they’re making missteps in clinical care. Screening vulnerable patients, asking about diet and supplement use, and providing clear guidance on oxalate moderation could prevent years of suffering and secondary illness.
It’s time to move oxalate awareness out of the shadows into mainstream medical practice — where it belongs.
References
Bhandari, M. R., & Kawabata, J. (2004). Assessment of antinutritional factors and bioavailability of calcium and zinc in wild yam (Dioscorea spp.) tubers of Nepal. Food Chemistry, 85(2), 281–287.
Bradbury, J. H., & Nixon, R. W. (1998). The acridity of raphides from the edible aroids. Journal of the Science of Food and Agriculture, 76(4), 608–616. (Nature’s Poisoned Spear)
Chen, Z., Ye, Z., Zeng, L., & Yang, W. (2003). Clinical investigation on gastric oxalate absorption. Chinese Medical Journal, 116(11), 1749–1751.
Diercks, B.-P. (2024). The importance of Ca2+ microdomains for the adaptive immune response. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research, 1871(5), 119710.
Diamantstein, T., & Odenwald, M. V. (1974). Influence of calcium ions on the proliferation and differentiation of antibody-producing cells. Immunology, 26(3), 509–514.
Elferink, J. G. (1987). Mode of activation of the metabolic burst in polymorphonuclear leukocytes by calcium oxalate crystals. Agents and Actions, 22(3–4), 295–301.
Elferink, J. G., & Deierkauf, M. (1987). Enzyme release from polymorphonuclear leukocytes during interaction with calcium oxalate microcrystals. The Journal of Urology, 138(1), 164–167.
Elferink, J. G., & Riemersma, R. C. (1980). Calcium oxalate crystal-induced cytolysis in polymorphonuclear leukocytes and erythrocytes. Agents and Actions, 10(5), 439–444.
Ermer, T., Eckardt, K. U., Aronson, P. S., & Knauf, F. (2016). Oxalate, inflammasome, and progression of kidney disease. Current Opinion in Nephrology and Hypertension, 25(4), 363–371.
Erickson, S. B., Cooper, K., Broadus, A. E., Smith, L. H., Werness, P. G., Binder, H. J., & Dobbins, J. W. (1984). Oxalate absorption and postprandial urine supersaturation in an experimental human model of absorptive hypercalciuria. Clinical Science, 67(2), 131–138.
Hatch, M., Mulgrew, S., Bourke, E., Keogh, B., & Costello, J. (1980). Effect of megadoses of ascorbic acid on serum and urinary oxalate. European Urology, 6(3), 166–169.
Hatch, M. (1998). Oxalate, hyperoxaluria, and intestinal injury. Urological Research, 26(5), 355–362.
Holloway, W. D., Argall, M. E., Jealous, W. T., Lee, J. A., & Bradbury, J. H. (2002). Organic acids and calcium oxalate in tropical root crops (world).
Holmes, R. P., Goodman, H. O., & Assimos, D. G. (2001). Contribution of dietary oxalate to urinary oxalate excretion. Kidney International, 59(1), 270–276.
James, L. F. (1972). Oxalate Toxicosis. Ctx Clinical Toxicology, 5(2), 231–243.
Konno, K., Inoue, T. A., & Nakamura, M. (2014). Synergistic defensive function of raphides and protease through the needle effect. PLoS One, 9(3), e91341.
Knight, J., Jiang, J., Assimos, D. G., & Holmes, R. P. (2006). Hydroxyproline ingestion and urinary oxalate and glycolate excretion. Kidney International, 70(11), 1929–1934.
Knight, J., Madduma-Liyanage, K., Mobley, J. A., Assimos, D. G., & Holmes, R. P. (2016). Ascorbic acid intake and oxalate synthesis. Urolithiasis, 44(4), 289–297.
Kumar, R., Lee, S., Minard, C. G., et al. (2021). Dietary oxalate induces mitochondrial dysfunction and modulates immune response in monocytes and macrophages: A pilot study in humans. Frontiers in Immunology, 12, 638582.
Lin, W. V., Turin, C. G., McCormick, D. W., Haas, C., & Constantine, G. (2019). Ascorbic acid-induced oxalate nephropathy: A case report and discussion of pathologic mechanisms. CEN Case Reports, 8(1), 67–70.
Marengo, S. R., Chen, D. H., Kaung, H. L., & Evan, A. P. (2013). Oxalate-induced oxidative stress in experimental models: Tissue deposition beyond the kidney. Urological Research, 41(1), 23–30.
Mashour, S., Turner, J. F., & Merrell, R. (2000). Acute renal failure, oxalosis, and vitamin C supplementation—A case report and review of the literature. Chest, 118(2), 561–563.
Perera, C. O., Hallett, I. C., Nguyen, T. T., & Charles, J. C. (1990). Calcium oxalate crystals: The irritant factor in kiwifruit. Journal of Food Science, 55(4), 1066–1069.
Robijn, S., Hoppe, B., Vervaet, B. a., D’Haese, P. C. & Verhulst, A. (2011). Hyperoxaluria: a gut-kidney axis? Kidney Int 80, 1146–1158
Savage, G. P., & Dubois, M. (2006). The effect of soaking and cooking on the oxalate content of taro leaves. International Journal of Food Sciences and Nutrition, 57(5–6), 376–381.
Scimeca, J. A., & Abernethy, R. H. (1979). Calcium oxalate crystals in pineapple tissue. Journal of Agricultural and Food Chemistry, 27(5), 1099–1101.
Simpson, G. L., & Ortwerth, B. J. (2000). The non-oxidative degradation of ascorbic acid under physiological conditions. Biochimica et Biophysica Acta, 1501(1), 12–24.
Sun, X.-Y., Ouyang, J.-M., Zhu, W.-Y., Li, Y.-B., and Gan, Q.-Z. (2015). Size-dependent toxicity and interactions of calcium oxalate dihydrate crystals on Vero renal epithelial cells. J. Mater. Chem. B 3, 1864–1878.
Suryavanshi, M. V., Bhute, S. S., Jadhav, S. D., Bhatia, M. S., & Shouche, Y. S. (2016). Hyperoxaluria leads to gut microbial dysbiosis in stone formers. BioMed Research International, 2016, 1–9.
Wiessner, J.H., Hasegawa, A.T., Hung, L.Y., Mandel, G.S., and Mandel, N.S. (2001). Mechanisms of calcium oxalate crystal attachment to injured renal collecting duct cells. Kidney Int. 59, 637–644.)